
INTRODUCTION
- Soap and detergents both are cleansing agents.
- The substances which remove the dust and, in water have the cleansing action are known as cleansing agents.
SOAPS
- Sodium and potassium salts of long-chain carboxylic acids are the molecules of soaps.
- Sodium salts of fatty acids are known as hard soaps and potassium salts of any fatty acids are known as soft soaps.
- As you all know that most of the dirt is oily in nature and oil does not dissolve in water.
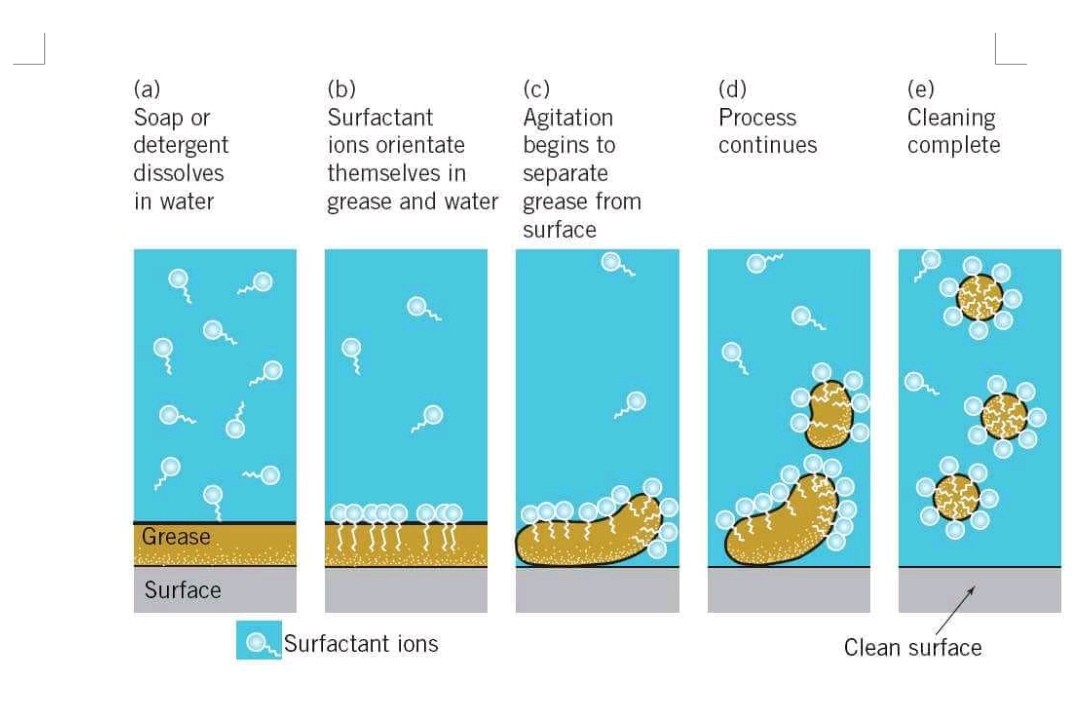
- Soap’s ionic end dissolves in water whereas, the carbon chain is dissolved in oil. Thus, the structure has been formed by the soaps known as micelles. Where the ionic end of the molecule is facing outside and the other end is towards the oil droplet. Thus, forming an emulsion in water.
- The micelle of soap thus helps us in washing the clothes as it dissolves the dirt in the water.
MICELLES –
- Soap has two ends and both the ends have different properties, one of them is hydrophilic that means it loves water and hence dissolves in water. Whereas the other end is hydrophobic that means hates water and hence dissolves in hydrocarbons.
- When at the surface of the water, soap is there, the hydrophobic tail of soap will not dissolve in water and the soap will align along the surface of the water with the ionic end in water and the hydrocarbon tail protruding out of water.
- The hydrocarbon portion of the soap is aligned outside in the water because of the unique orientation of these molecules.
- By the formation of clusters of molecules in which the hydrophobic tails are inside of the cluster and the ionic ends are on the surface of the cluster, this formation is achieved which is known as a micelle.
- In the form of a micelle, the soap is able to clean, since the oily dirt will be collected in the mid-Centre of the micelle.
PREPARATION OF SOAP –
- Soaps that are made from sodium are prepared from heating fat with the aqueous solution of sodium hydroxide. This process is known as saponification.
- In the process of saponification, the esters of fatty acids are being hydrolyzed whereas, in the colloidal form the soap is obtained.
- After this, it is precipitated by adding sodium chloride to the solution.
- Only the sodium and potassium soaps are soluble in water and can be used for cleaning purposes.
- When we compare, the potassium soaps are softer to the skin than sodium soaps.
WHY DO SOAPS DON’T WORK IN HARD WATER?
- Calcium and magnesium ions are present in hard water. These ions form insoluble calcium and magnesium soaps respectively when the soaps are dissolved in them.
- Soaps become useless because the insoluble soaps get separated as scum in the water. It acts as a hindrance to the cleansing because these precipitates stick on the fiber and act as a gummy mass on the cloth.
DETERGENTS
- The problem of not being able to use soaps for washing in hard water can be overcome by the use of detergents as a cleansing agent. These are the substances that have all the properties of soap but do not contain any soap.
- These cleansing agents can be used in both hard as well as soft water.
- They do not make any insoluble precipitate with the ions of magnesium and calcium in hard water.
- Are usually sulphonate and ammonium salts of long-chain carboxylic acids.
- Usually used to make shampoos and products used for cleaning clothes.
- These are classified into three – anionic detergents, cationic detergents, and non-ionic detergents.



